

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
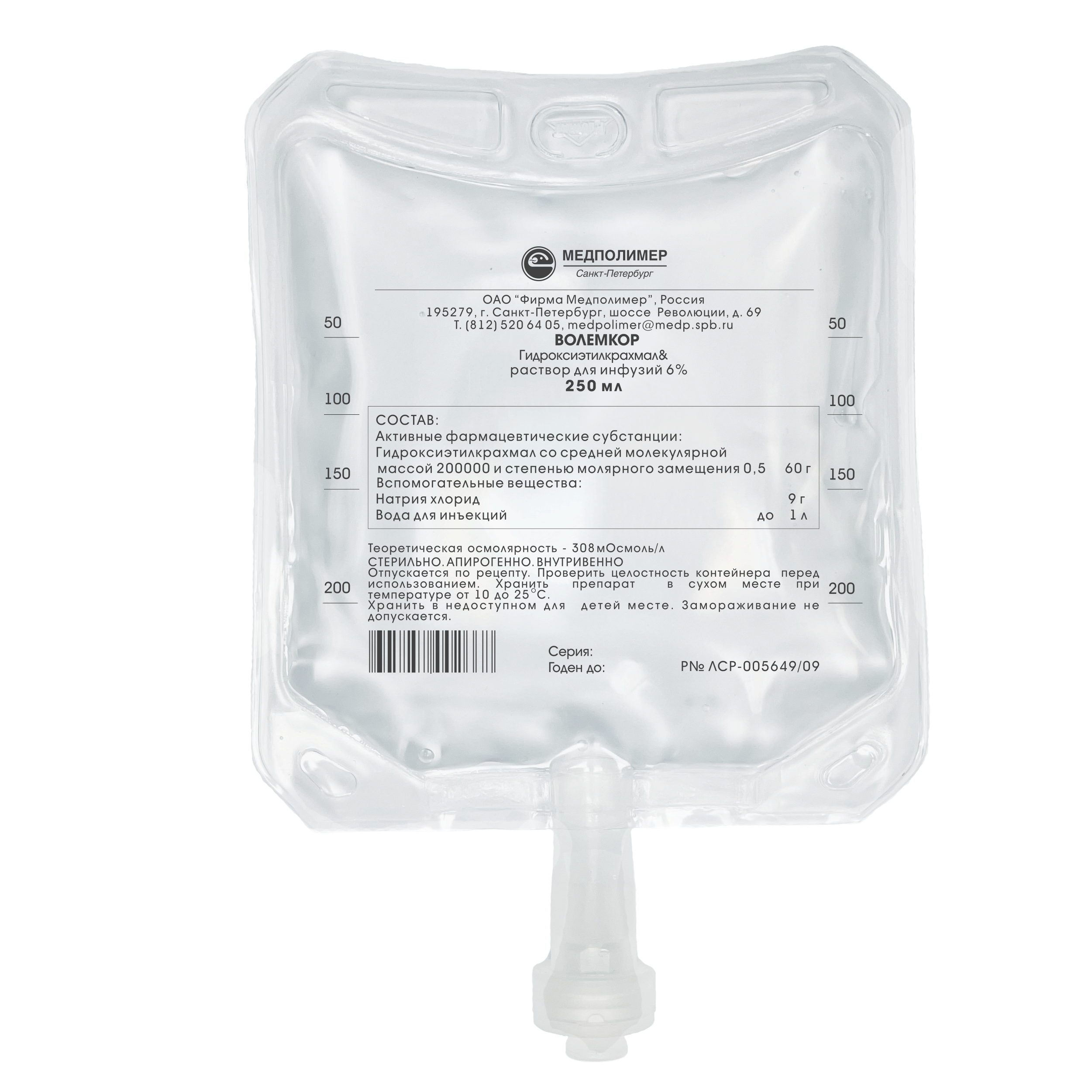
Marketing authorisation No. LSR-005649/09
Composition:
Hydroxyethyl starch
with an average molecular weight of 200000
and a substitution degree of 0.5 – 60.0 g,
Sodium chloride – 9.0 g,
Water for injections – up to 1.0 L.
Theoretical osmolarity – 308 mOsmoI/L.
Therapeutic class: plasma volume expander
Pharmacological effect. Volemcor is a colloidal plasma replacement solution based on hydroxyethylated starch (HES), a high molecular weight compound consisting of polymerized dextrose residues. The source of HES is native starch (amylopectin), which is split to give molecules with definite mol mass, and also hydroxyethylation, during which free hydroxyl groups of dextrose residues are substituted by C2/C6 hydroxyethyl groups. HES undergoes prolonged hydrolysis by serum amylase to form oncotically active oligo- and polysaccharides of various molecular weights. Volemcor, 6 % solution for infusions – HES preparation (pentastarch) with the average molecular weight of 200,000 Da and a substitution degree of about 0.5.
Due to the ability to bind and retain water it has a volemic effect that is the ability to increase the circulating blood volume (CBC) by 85-100% of the administered volume, which is maintained in steady-state for 4-6 hours. It has a plasma-substituting effect, restores impaired hemodynamics, improves microcirculation, blood rheological properties (by reducing hematocrit index), reduces plasma viscosity, reduces platelet aggregation and prevents erythrocyte aggregation. The similarity of the structure of HES with that of glycogen explains the high level of tolerability and almost complete absence of adverse reactions.
Pharmacokinetics. After intravenous administration it is excreted renally (in 24 hours–about 70% of the administered dose of penta starch) and via the bile. A small amount accumulates in the reticulo-endothelial system (without toxic effects on the liver, lungs, spleen and lymph nodes), where it is broken down by amylase and subsequently excreted renally and via the intestine.
Indications. Treatment of hypovolemia in patients with acute blood loss, if use of crystalloid solutions is insufficient.
Shelf life: 2 years.