

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
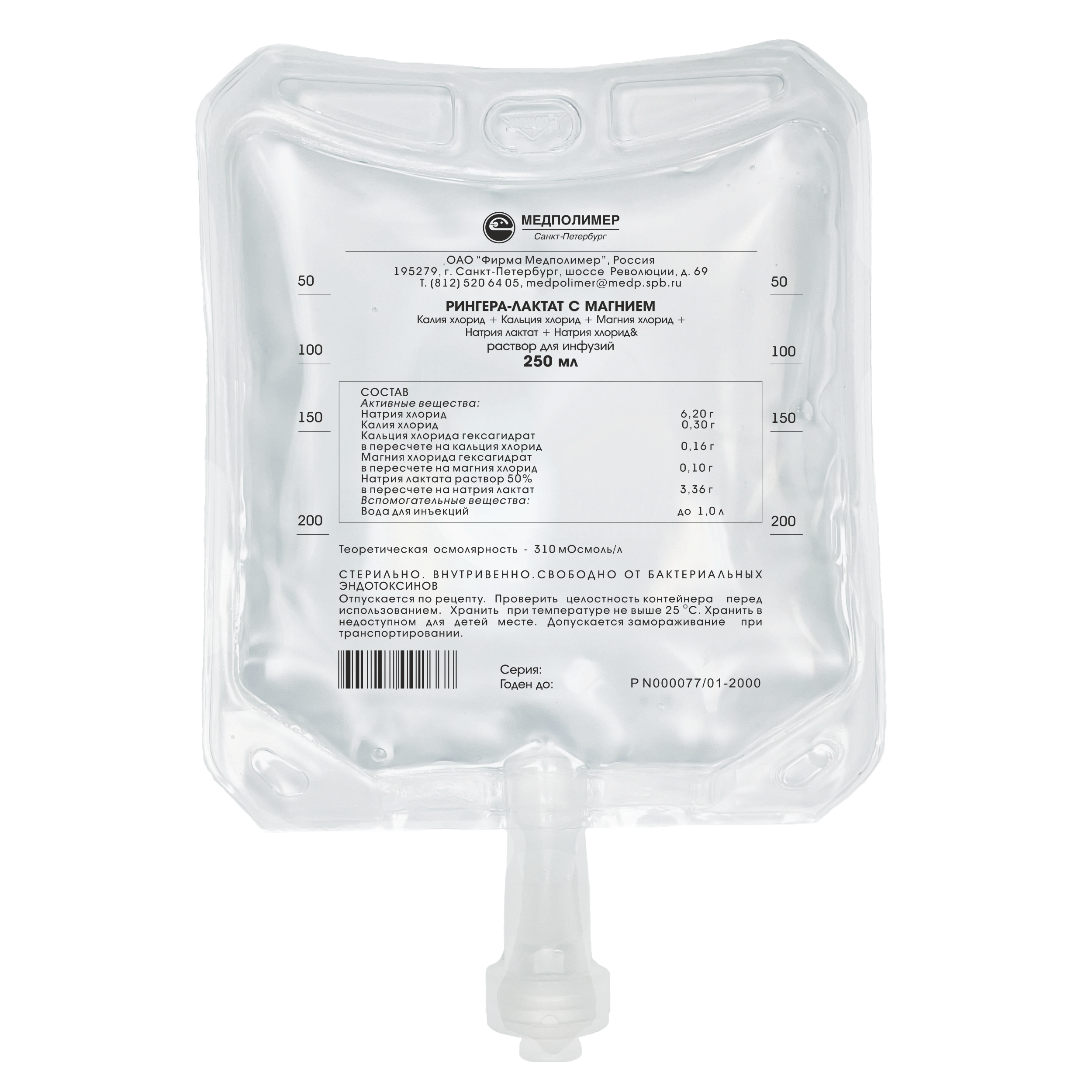
Marketing Authorisation No. P N000077/01
Composition:
Sodium chloride – 6.20 g,
Potassium Chloride — 0.30 g,
Calcium chloride (on dry basis) - 0.16 g,
Magnesium chloride (on dry basis) - 0,10 g,
Sodium acetate (on dry basis) - 3.36 g,
Water for injections – up to 1.0 L.
Theoretical osmolarity — 283 mOsmoI/L.
Therapeutic class: electrolyte rebalancing agent
Pharmacological properties. Combination drug, eliminates disorders of fluid and electrolyte balance (with correction of metabolic acidosis by changing the buffer capacity of blood). It improves rheological properties of blood and peripheral circulation (as a result of hemodilution and lowering of blood viscosity). It has plasma-substituting, detoxifying and diuretic effects. When administered parenterally, Mg2+ ions have anticonvulsant, antiarrhythmic, hypotensive, relaxing effect, they regulate metabolism, inter-neuronal transmission and muscle excitability, prevents Ca2+ ions from entering the presynaptic membrane, reduces the amount of acetylcholine in the peripheral nervous system and central nervous system. Faciliates smooth muscle relaxation, blood pressure lowering (mostly elevated), increases diuresis.
Indications. Impaired circulation with underlying water depletion and/or fluid outflow out of the blood stream into the extracellular space: trauma, burns, haemorrhagic, traumatic, operative and post-operative shock, peritonitis, intestinal obstruction, diarrhoea of various origin, metabolic acidosis.
Shelf life: 2 years.