

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
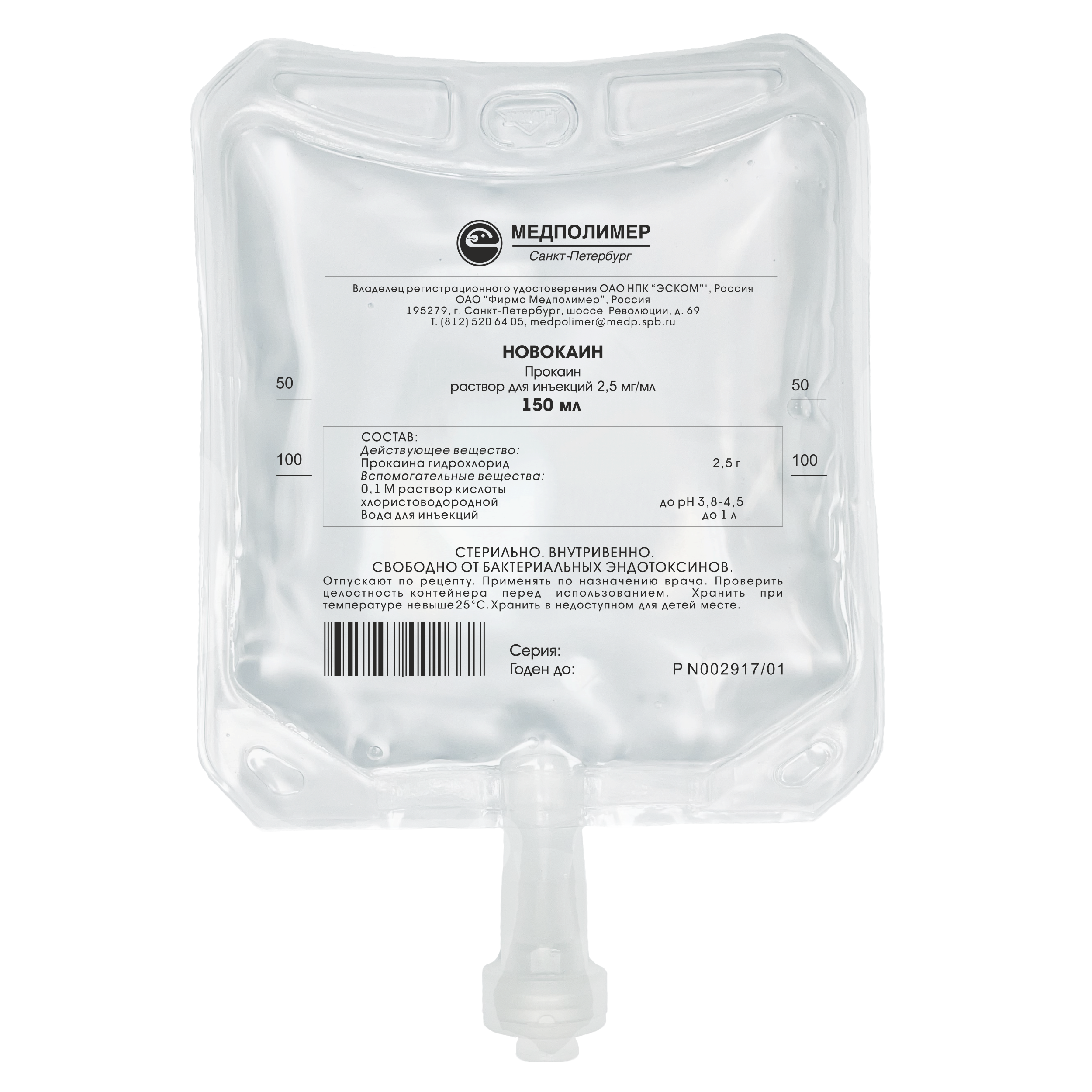
Marketing Authorisation No. P N002917/01
Composition:
Procaine hydrochloride — 2.5 g or 5 g,
0.1 M hydrochloric acid solution — pH up to 3.8-4.5,
Water for injections – up to 1 ml
Therapeutic class: local anesthetic agent.
Pharmacological effect:
Pharmacodynamics. A local anesthetic with moderate anesthetic activity and a wide therapeutic window. Being a weak base, it blocks sodium channels, prevents generation of impulses in the afferent nerve endings and conduction of impulses along the nerve fibers. It inhibits not only pain impulses, but also impulses of different modality.
When administered intravenously it has analgesic, antishock, hypotensive and antiarrhythmic effect (increases effective refractory period, reduces excitability, automaticity and conduction), in high doses it may disrupt neuromuscular conduction.
Pharmacokinetics. Subject to complete systemic absorption. The degree of absorption depends on the site and route of administration (especially vascularisation and blood flow velocity in the area of administration) and the resulting dose (quantity and concentration). It is rapidly hydrolyzed by plasma and hepatic esterases to form 2 major pharmacologically active metabolites: diethylaminoethanol (has a moderate vasodilator effect) and para-aminobenzoic acid.
The elimination half-life (T1/2) is 30-50 seconds, in the neonatal period it is 54-114 seconds.
It is excreted mainly renally as metabolites, not more than 2% is excreted unchanged.
Indications: Infiltration (incl. intraosseous), conduction, epidural, spinal anaesthesia; terminal (superficial) anaesthesia (in otorhinolaryngology); vagosympathetic cervical, paranephral, circular and paravertebral blockages.
Shelf life: 3 years.