

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
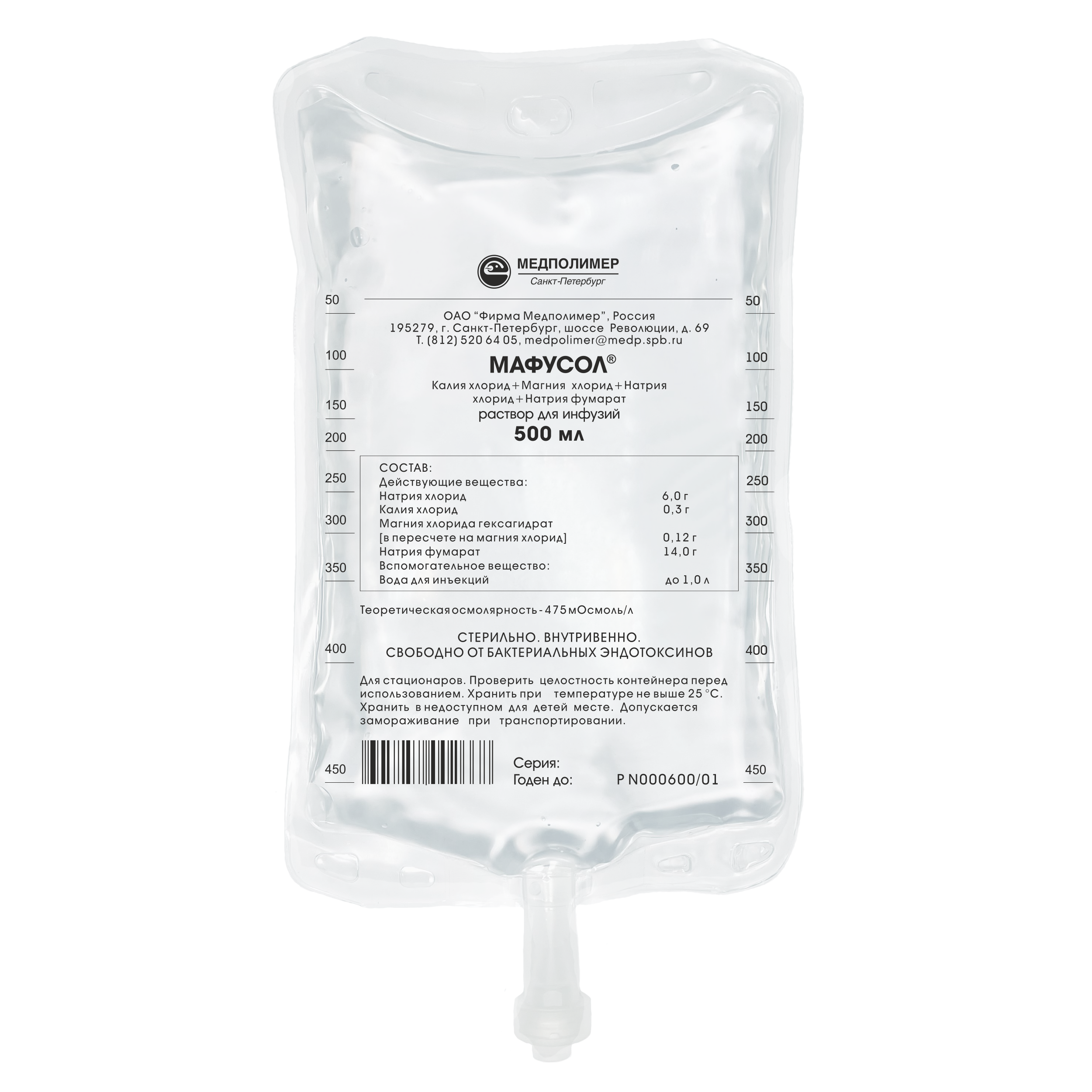
Marketing Authorisation No.: PN 000600/01
Composition:
Sodium chloride – 6.0 g,
Potassium Chloride — 0,3 g,
Magnesium chloride – 0.12 g,
Sodium fumarate — 14.0 g
Water for injections – up to 1.0 L.
Theoretical osmolarity — 475 mOsmoI/L.
Therapeutic class: rehydrating agent.
Pharmacological properties. Combination drug acting as anti-oxidant, rehydrating, antiaggregant, diuretic and detoxifying agent. Activates adaptation of the cells to oxygen deficiency; quickly replenishes circulating blood volume in hypovolemic states, prevents tissue dehydration, reduces blood viscosity, improves its rheological properties, demonstrates hemodynamic effect.
Indications. Hypovolemia and hypoxia (blood loss, shock, trauma, poisoning). Acute cerebrovascular events of ischemic and hemorrhagic type in adults. As a perfusion component to fill the circuit of the artificial blood-circulation apparatus in cardiac surgery in adults and children.
Dosage and Administration. Mafusol is administered intravenously or intra-arterially. The dose and rate of the drug infusion are selected according to the indication and condition of the patient. In mild or moderate shock in adults (haemorrhagic, burn, traumatic, operative) Mafusol is administered at a dose of 2-3 liters, initially by bolus infusion, and when hemodynamic parameters are normalized — by IV drip; in children at a dose of 20-25 ml/kg.
It is recommended to use Mafusol in combination with erythrocyte-containing media as well as colloid haemodynamic blood substitutes for severe shock in adults. Mafulsol dose in this case is selected individually, but not less than 1 litre; children are administered at least 15 ml/kg. In severe intoxications in adults (peritonitis, septicaemia, intestinal obstruction, etc.) infuse up to 2-3 litres/day in combination with other detoxifying agents; in children —at a dose of 30-35 ml/kg/day.
As a haemodiluent in the heart-lung apparatus, Mafusol can make up to 50% of the perfusion solution injected into the apparatus.
In case the blood loss is not exceeding 15% of the total blood volume in adults and children, the drug product may be used as the only infusion medium. Mafusol can be administered instead of other saline infusion solutions.
Drug-drug interaction Mafusol can be used in combination with colloid solutions (polyglucin, rheopolyglucin, neohaemodes, haemodez, gelatinol, etc.); the drug is also compatible with onated blood, erythromass, plasma and other blood products.
Mafusol does not prevent the use of commonly used antishock agents, including: drugs for neuroleptanalgesics (fentanyl, droperidol), benzodiazines (diazepam etc.), as well as myorelaxants (suxamethonium etc.), proteolysis inhibitors (aprotinin) and adrenomimetic agents (dopamine, epinephrine).
Shelf life: 3 years.