

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
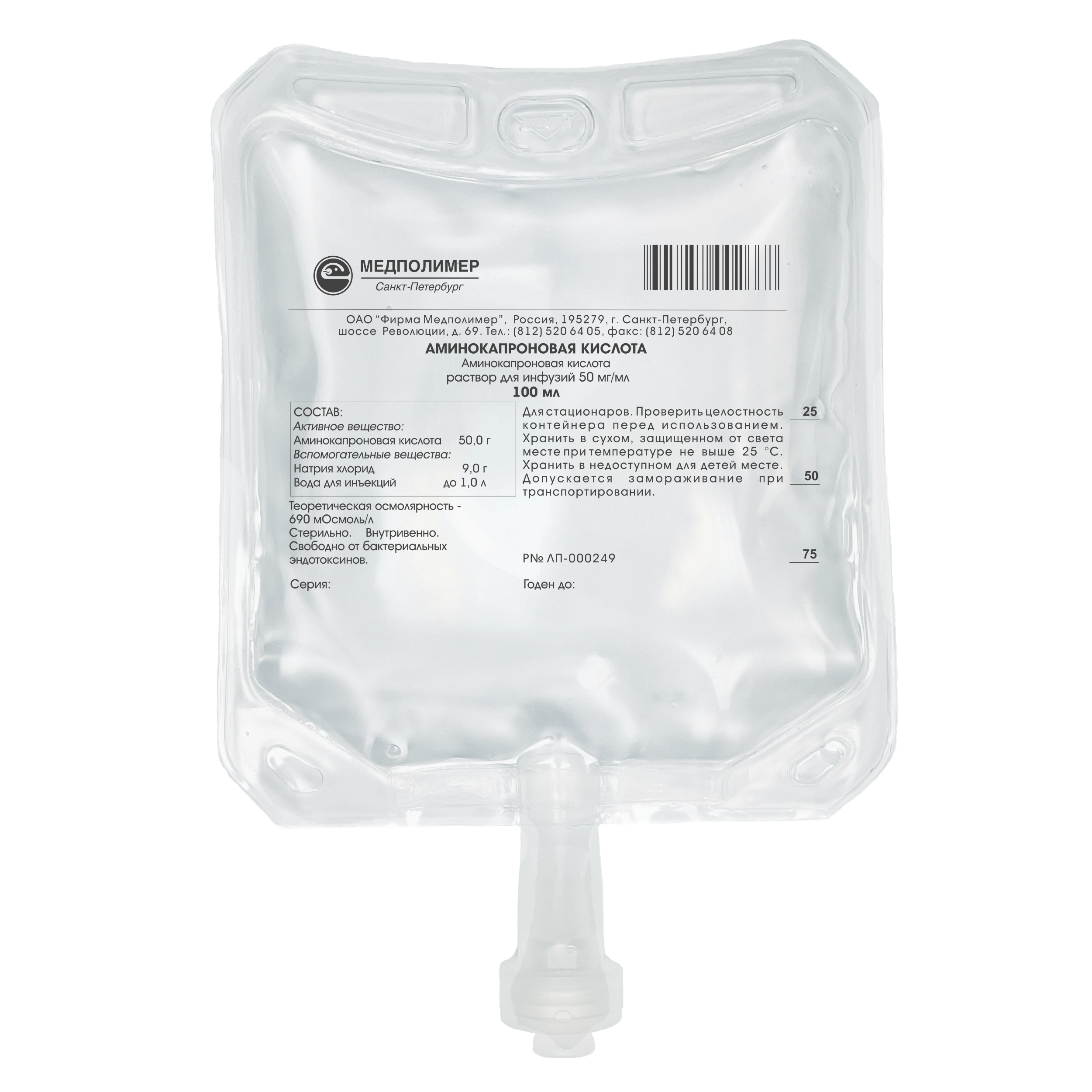
Marketing Authorisation No.: LP-000249
Composition:
Aminocaproic acid — 50.0 g,
Sodium chloride – 9.0 g,
Water for injections – up to 1.0 L.
Theoretical osmolarity — 690 mOsmoI/L.
Therapeutic class: hemostatic agent, fibrinolysis inhibitor.
Pharmacological properties.
Pharmacokinetics. When administered intravenously, the effect exhibits within 15-20 minutes. The drug is rapidly excreted renally: 4 hours after administration 40-60% of the dose is excreted intact to urine. In excretory function of the kidneys abnormality, aminocaproic acid blood concentration increases significantly.
Pharmacodynamics. Aminocaproic acid is a lysine synthetic analogue. It inhibits fibrinolysis by competitively saturating the lysine-binding receptors through which plasminogen (plasmin) binds to fibrinogen (fibrin). The drug also inhibits biogenic polypeptide, kinins, (inhibits activating effect of streptokinase, urokinase, tissue kinases on fibrinolysis), neutralizes the effects of kininogenase, trypsin and hyaluronidase, reduces capillary permeability. Aminocaproic acid has antiallergic activity, enhances the detoxification function of liver and inhibits antibody formation.
Indications. Bleeding (hyperfibrinolysis, hypo- and afibrinogenemia), bleeding during surgery and morbid conditions, accompanied by increased fibrinolytic activity of blood (during neurosurgical, intracavitary, thoracic, gynecological and urological surgeries, including procedures on the prostate, lungs, pancreas; tonsillectomy, following dental interventions, during surgeries with the use of a heart-lung machine). Internal medicine diseases with hemorrhagic syndrome; premature placental abruption, complicated abortion. Also used to prevent secondary hypofibrinogenemia during profuse transfusions of preserved blood.
Shelf life: 3 years.