

About Us
Manufacture
Products
Procurement
Working at Medpolymer
- Contact Us
- Ru
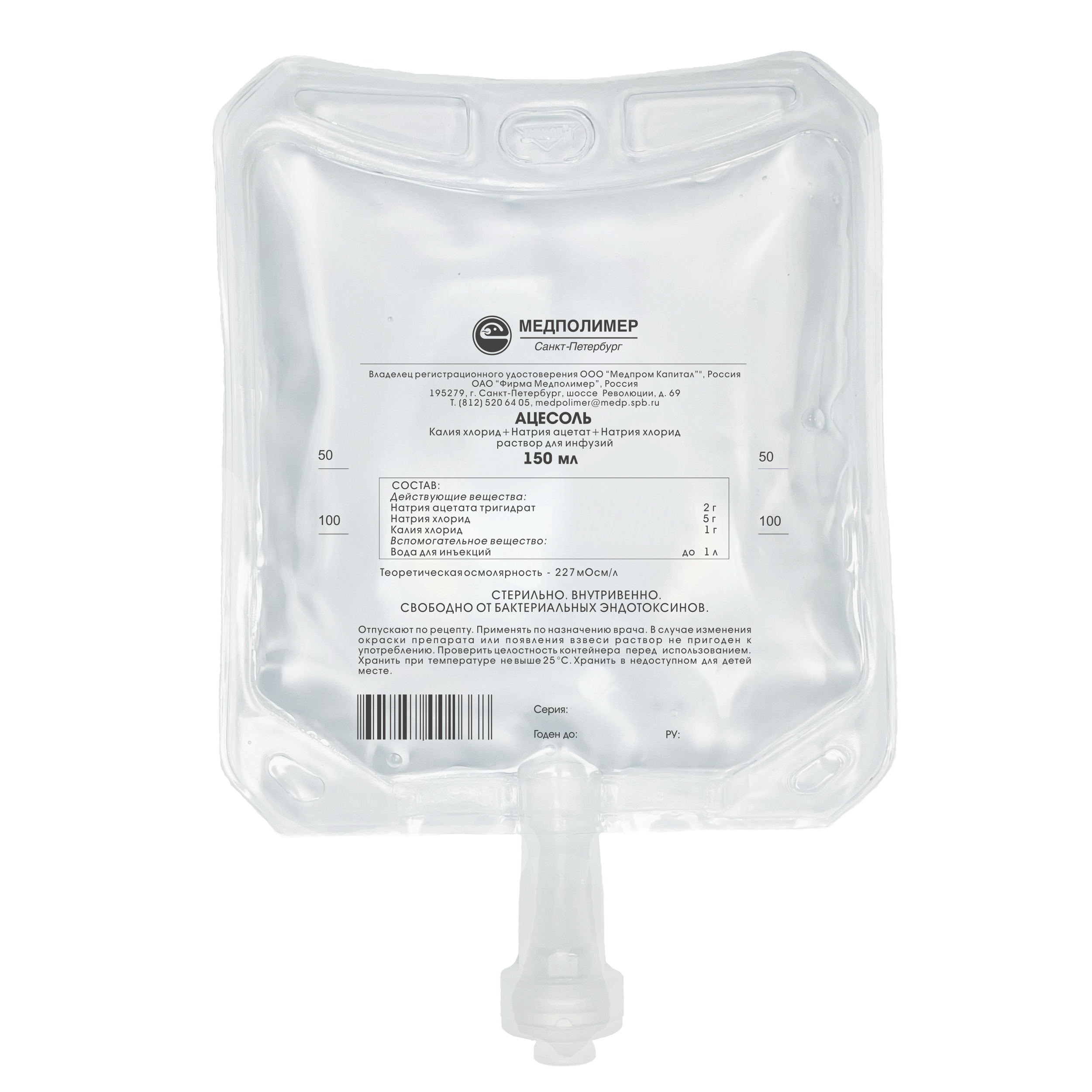
Marketing Authorisation No. P N001120/01
Composition:
Sodium acetate trihydrate — 2 g,
Sodium chloride — 5 g,
Potassium Chloride — 1 g,
Water for injections —up to 1.0 L.
Theoretical osmolarity – 227 mOsmoI/L
Therapeutic class: Rehydrating agent.
Pharmacological effect.
Pharmacodynamics. The combination drug has a detoxification, plasma-substituting, rehydrating, diuretic, antishock and antiplatelet action. It has a haemodynamic effect, reducing hypovolemia, preventing hemoconcentration and the development of metabolic acidosis, improving capillary circulation and stimulates diuresis.
Pharmacokinetics. K+ and Na+ ions are briefly retained in the blood flow and are rapidly distributed throughout all tissues of the body. Excreted mainly renally, and in small amounts through the intestinal tract, sweat, tears, and etc.
In the body, acetate is activated to acetyl-coenzyme A (acetyl-CoA), then the basic quantity of the active acetate is completely oxidized to carbon dioxide and water in the Krebs cycle. Acetate is oxidized in muscle cells, therefore the body's ability to metabolize it mainly depends on lean body mass. The metabolism of acetyl-CoA may also follow a minor oxidation pathway to form fatty acids, keto acids and cholesterol.
Indications. Dehydratation, intoxication with underlying water depletion (including cholera, acute shigellosis, food toxicoinfection).
Shelf life: 3 years.